"IVD" is the English abbreviation for in vitro diagnostic products. In the
international arena, IVD, as a sole branch of medical devices, has its unique
definition and regulatory system, especially in the United States Food and Drug
Administration (FDA) and the European Union (EC). In vitro diagnostic products
are those reagents, instruments, and systems intended for use in diagnosis of
disease or other conditions, including a determination of the state of health,
in order to cure, mitigate, treat, or prevent disease or its sequelae. Whereas
it is different in China, that IVD is not distinguished and defined solely. In
other words, there is no concept of IVD existing in China. In China, the normal
contained products of IVD recognized internationally are broken up and
subordinated to medical devices (MD), in vitro diagnostic reagents (IVD
Reagents), as well as drugs respectively.
In China, except two kinds of in vitro diagnostic reagents intended for use in
blood source screening and radiolabeled defined as drugs, all of other IVD are
medical devices (MD).
However, based on actual needs, IVD reagents is distinguished and defined
solely. A set of provisions issued in April to May 2007 are related to
manufacturing, quality system, product registration for market approval and
distribution of postmarket control. IVD reagents are defined in section 3 of
Management Method of In Vitro Diagnostic Reagents Registration (Interim) taken
effect as of June 1, 2007.
In vitro diagnostic reagents that are administrated as medical devices, include
any reagent, kit, calibrator, quality control product (substance) and so on,
whether used alone or in combination with instrument, apparatus, appliance and
system, intended to be used in vitro for the examination of human specimens
taken from human body(various body fluid, cells and tissue samples, etc.) during
the prevention, diagnosis, treatment and sequelae observation of disease, health
evaluation as well as the prediction on hereditary disease.
Meanwhile based on the product risk, IVD reagents are classified into class III,
II and I. Moreover, some reagents are listed in each classification as example.
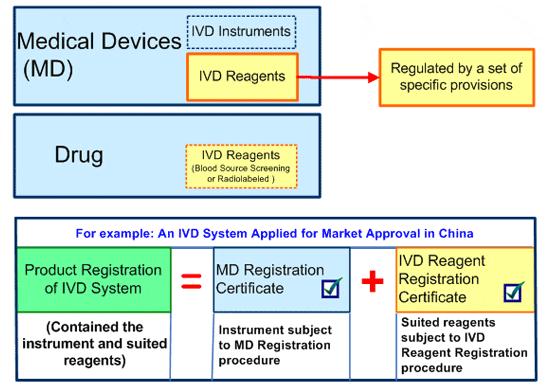
Therefore, IVD in China is subject to the whole set of MD requirements of
regulations and provisions, and there is no specific regulations aimed at IVD.
However IVD reagents define solely and are subject to not only a whole set of MD
regulations but also its specific requirements of provisions. For an IVD system
contained the instrument and suited reagents, two product registration
certificates have to be acquired for the market approval complied with two
different and absolute regulatory systems and procedures, Medical Device
Registration for the instrument and IVD Reagent Registration for suited
reagents.
